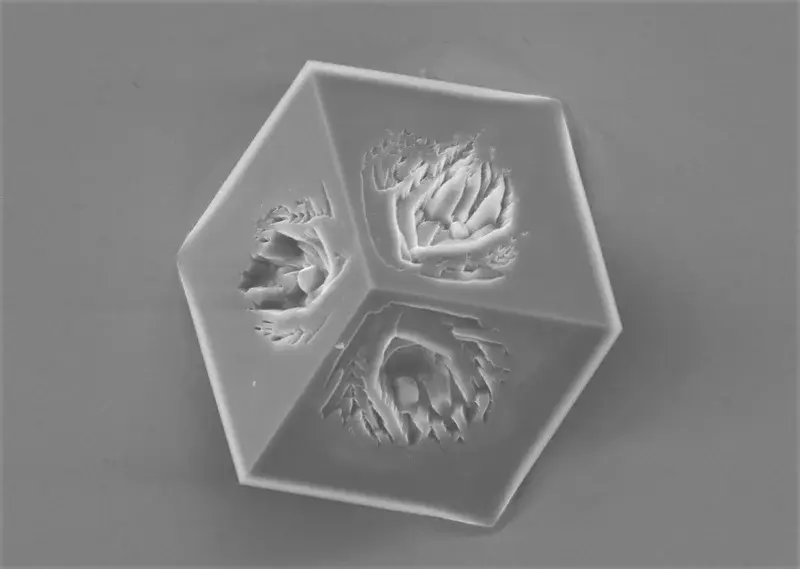
Wannabe cubic calcite
This imperfect cube of calcite was formed on the gold surface during the electrochemically assisted scaling process. Once negative electrochemical potential is applied to the gold surface, the oxygen dissolved in water undergoes reduction, yielding hydroxide anions. These anions accumulate at the gold-solution interface, forming a high pH layer. As calcite becomes less soluble with the increasing pH, it can precipitate on gold even from undersaturated solution. Like this wannabe cubic calcite crystal.
Categories
Location
Tags
Colours
Image properties
1281 × 911 px;
image/tiff; 1006.6 KB
Taken in
2022
Submitted on 1 December 2022
Licence
Creative Commons Attribution 3.0 Unported (CC BY 3.0)
Credit
Joanna Dziadkowiec (distributed via imaggeo.egu.eu)
Share
Appreciate
Report